AVENIO Tumor Tissue Surveillance Kit – Longitudinal Tumor Burden Monitoring - Roche Sequencing Store

Analytical performance evaluation of a commercial next generation sequencing liquid biopsy platform using plasma ctDNA, reference standards, and synthetic serial dilution samples derived from normal plasma. - Abstract - Europe PMC
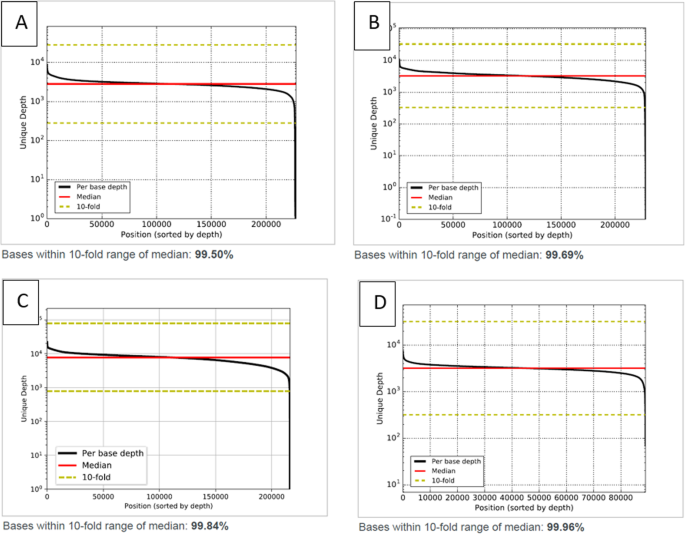
Analytical performance evaluation of a commercial next generation sequencing liquid biopsy platform using plasma ctDNA, reference standards, and synthetic serial dilution samples derived from normal plasma | BMC Cancer | Full Text

PDF) Analytical performance evaluation of a commercial next generation sequencing liquid biopsy platform using plasma ctDNA, reference standards, and synthetic serial dilution samples derived from normal plasma
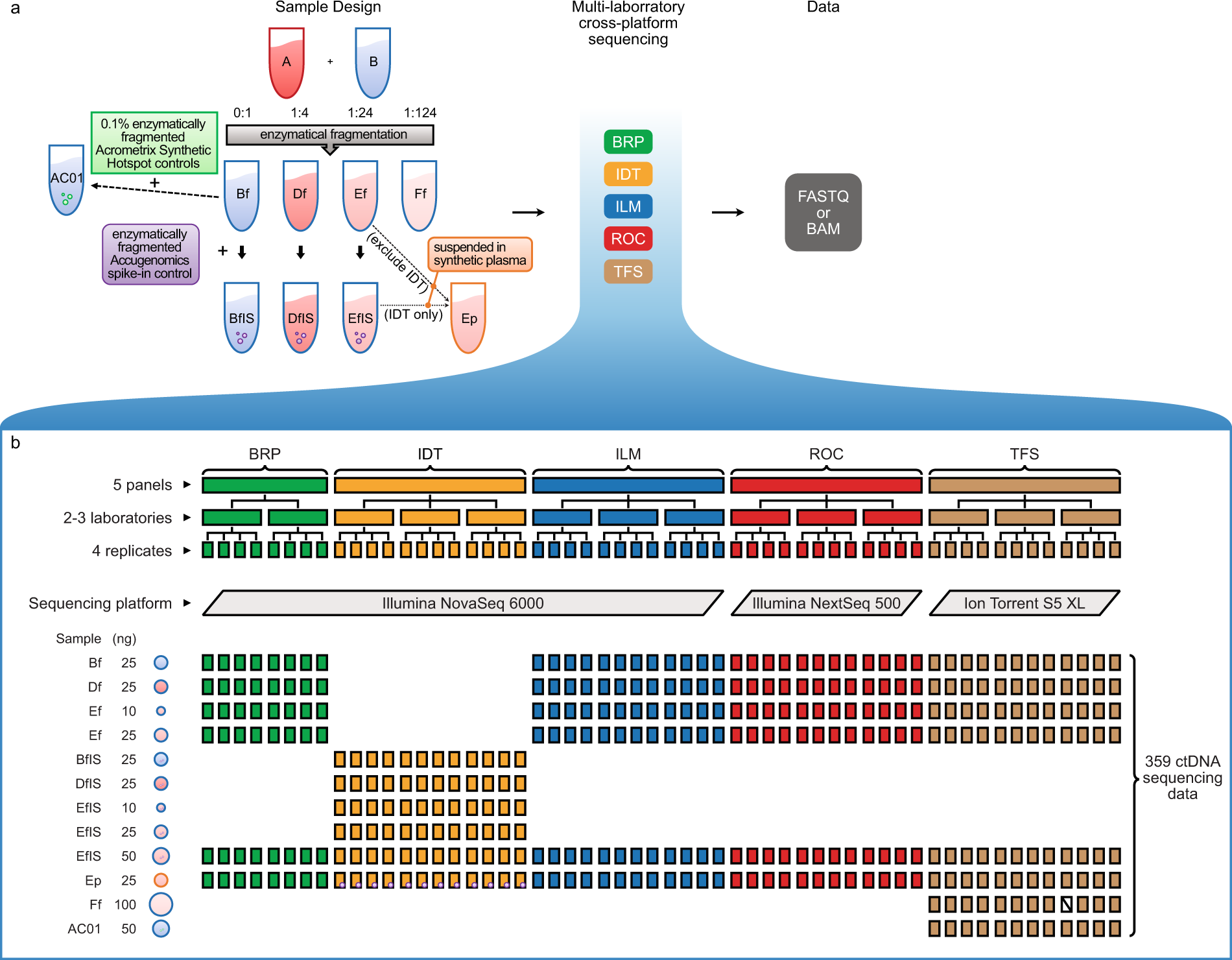
Ultra-deep sequencing data from a liquid biopsy proficiency study demonstrating analytic validity | Scientific Data

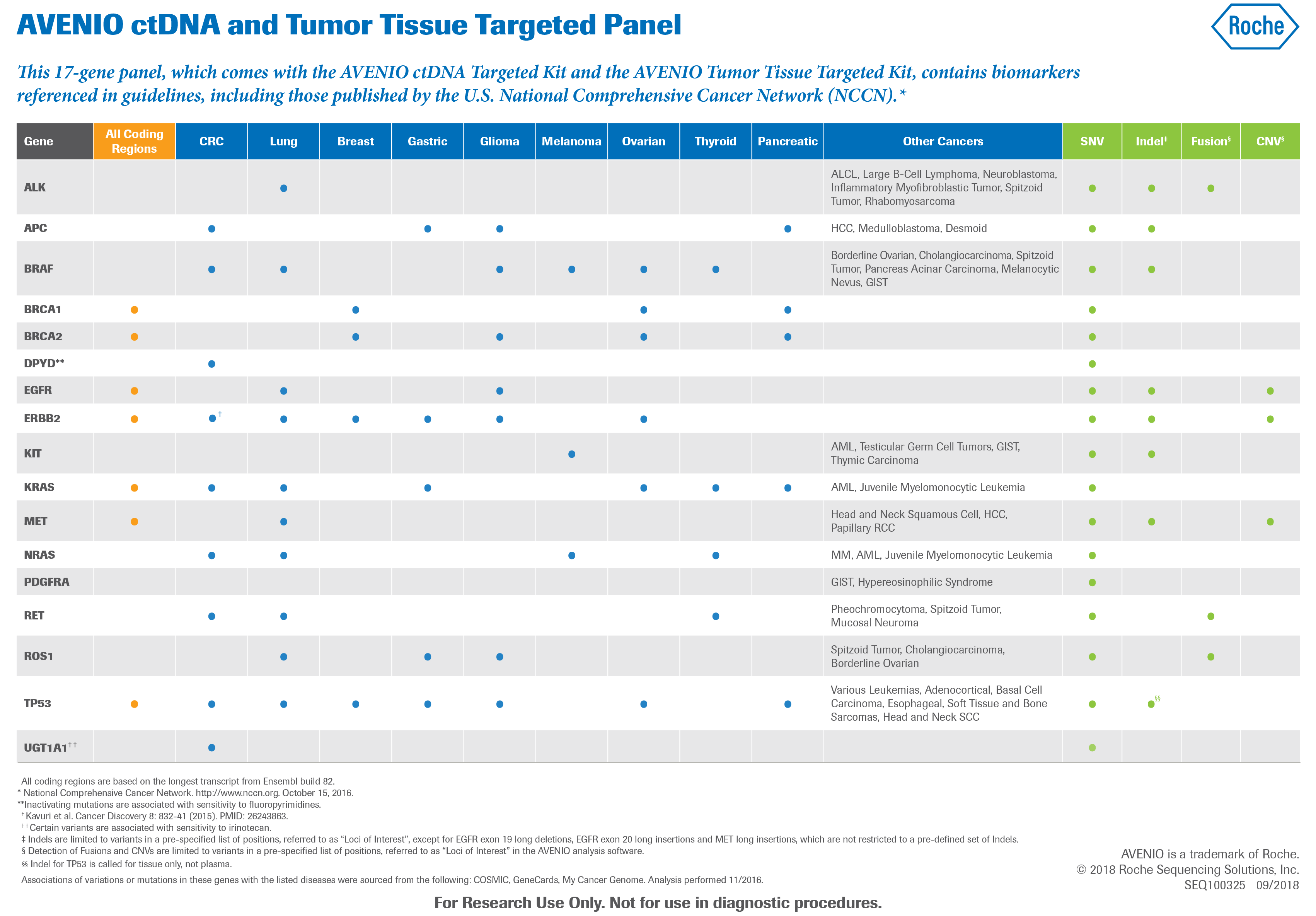
Kit di reagenti NGS - AVENIO - Roche Sequencing Solutions - da ricerca / per estrazione di DNA / per oncologia

Analytical performance evaluation of a commercial next generation sequencing liquid biopsy platform using plasma ctDNA, reference standards, and synthetic serial dilution samples derived from normal plasma | BMC Cancer | Full Text